See what’s in our product pipeline
We're investing in research and development to offer more solutions to challenges in transplant. Get to know our product pipeline and see our investment in the future.
Our future (in development)
We’re living up to our commitment to transform transplant medicine by leveraging our research and development capabilities to discover new therapies.
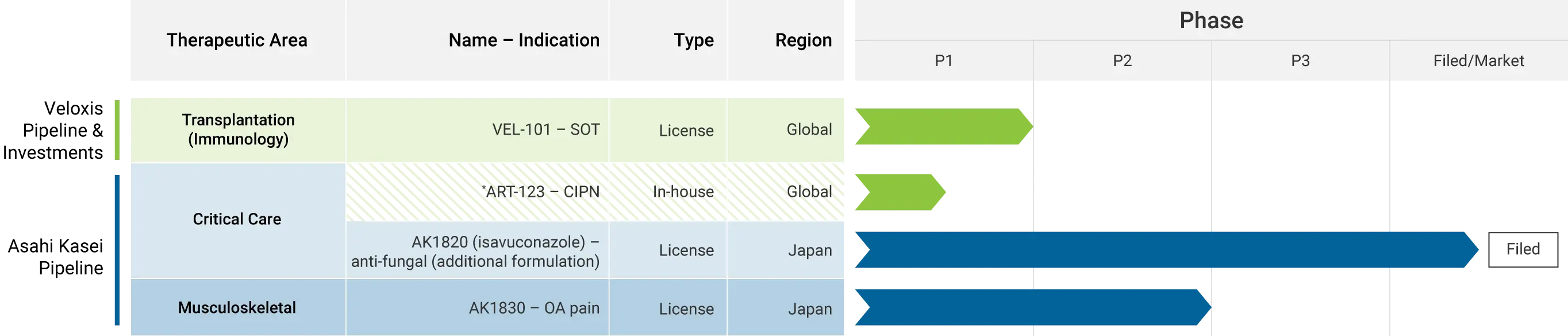
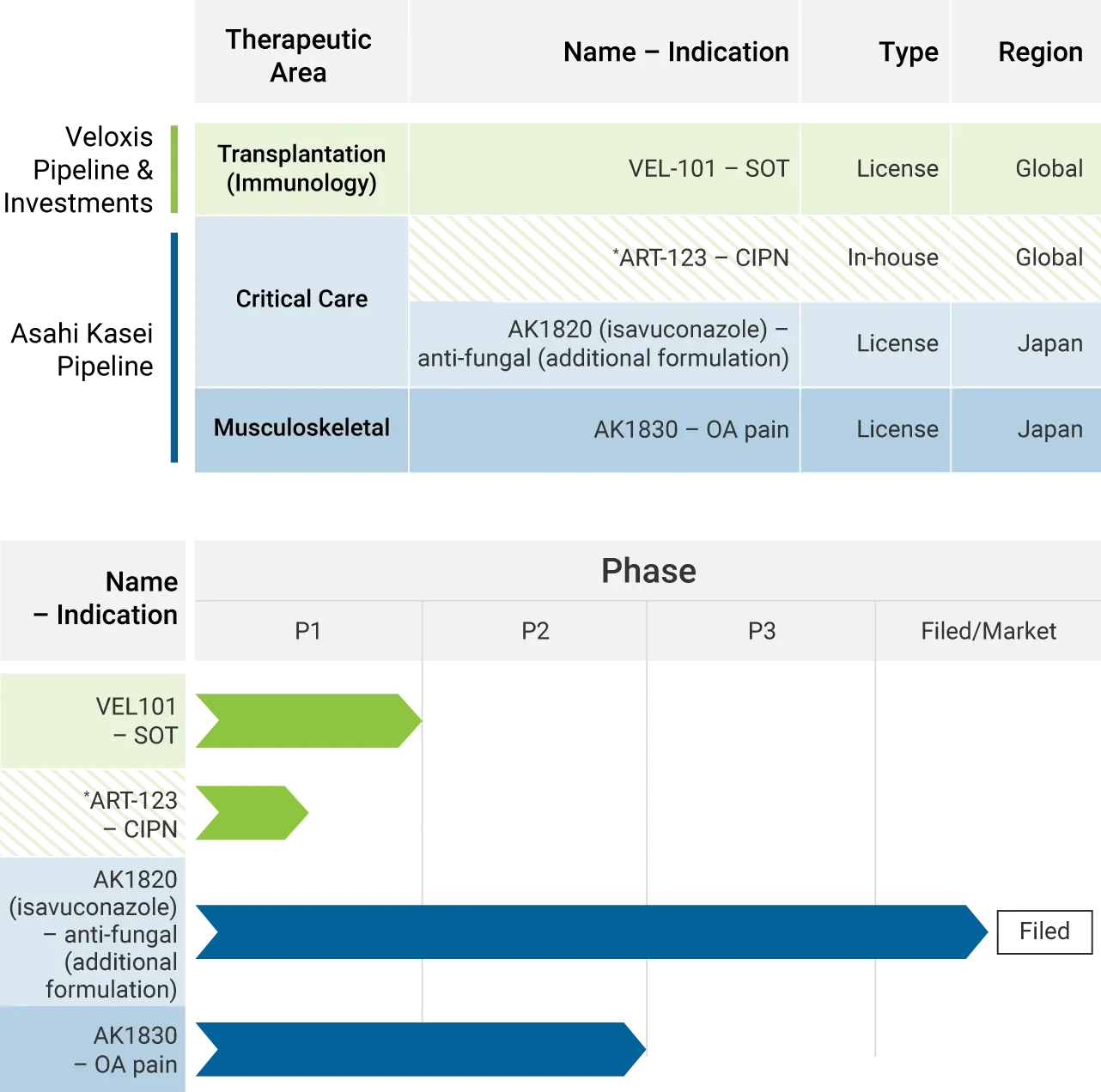
Veloxis Pipeline & Investments:
VEL101
- Therapeutic Area: Transplantation (Immunology)
- Name: VEL101
- Indication: SOT
- Type/Region: License/Global
- Phase: Phase 1
Asahi Kasei Pipeline:
*ART-123 – CIPN
- Therapeutic Area: Critical Care
- Name: ART-123
- Indication: CIPN
- Type/Region: In-house/Global
- Phase: Phase 1
AK1820 (isavuconazole)
- Therapeutic Area: Critical Care
- Name: AK1820 (isavuconazole)
- Indication: anti-fungal (additional formulation)
- Type/Region: License/Japan
- Phase: Filed/Market
AK1830
- Therapeutic Area: Musculoskeletal
- Name: AK1830
- Indication: OA pain
- Type/Region: License/Japan
- Phase: Phase 2
*Joint development program for ART-123 in US and Japan.
SOT=solid organ transplant; CIPN=chemotherapy-induced peripheral neuropathy; OA=osteoarthritis.
VEL-101 Clinical Program
VEL-101, a novel investigational immunosuppressant formerly known as FR104, is a monoclonal antibody fragment that inhibits costimulation via direct CD28 blockade.1 VEL-101 will be developed as a subcutaneous, at-home self-administration medication that may eliminate many of the toxicities seen with current standard of care immunosuppression.2
CD28 plays an essential role in T-cell activation, while CTLA-4 plays a key role in preventing or regulating T-cell activation.1,3,4 As a direct blocker of CD28 on the T cell, VEL-101 has a unique mechanism of action. Direct blockade of CD28 on the T cell with VEL-101 may result in inhibition of the stimulatory effects of CD28 while preserving the immunoregulatory effects of CTLA-4.1,3,4 As a result of VEL-101's unique mechanism of action, it will be studied as a maintenance immunosuppressant for solid organ transplant recipients.
Animal and initial human studies have been completed with VEL-101, and based on the results, the development program is continuing.1,3
Watch our video to learn more about VEL-101's proposed mechanism of action.
VEL-101 and Direct CD28 Costimulatory Blockade
VEL-101 IS AN INVESTIGATIONAL AGENT FOR WHICH THERE IS NO MARKETING AUTHORIZATION IN THE UNITED STATES. THE SAFETY AND EFFICACY OF VEL-101 HAS NOT BEEN ESTABLISHED. PROPOSED MOA DATA ARE BASED ON IN VITRO/IN VIVO DATA.
After an organ transplant, the immune system will target the transplanted organ for destruction.1
This involves antigen presentation by antigen-presenting cell to the T-cell receptor, known as signal 1, and costimulatory binding between the APC and T cell, known as signal 2.2
Activation of intracellular pathways triggers expression of inflammatory cytokines such as IL-2, providing signal 3 and resulting in T-cell activation, and proliferation.2
Costimulation, or signal 2, is essential in driving T-cell responses, via a complex network of signals.2
Among the best characterized and most important costimulatory interactions is CD80 and CD86 on antigen-presenting cells, with CD28 on T cells.3
CD28-mediated costimulation is integral to the immune response against a transplanted organ.3
Commonly used small molecule immunosuppressive agents act on intracellular targets. While effective, these strategies are associated with cardiometabolic, nephrotoxic, and neurotoxic side effects.2,4
Another approach to immunosuppression involves CD28 blockade through binding of CD80/86 molecules on APCs.2 This approach, however, additionally blocks CTLA-4, resulting in restoration of T-effector cell proliferation and function and may lead to allograft rejection.5,6
VEL-101, an investigational agent not yet approved for commercial use, is a pegylated monoclonal antibody fragment that specifically binds to CD28 on T cells.5
This action blocks the interaction of CD28 with CD80 and CD86, thereby attenuating T-cell activation and proliferation.5
By direct binding to CD28, VEL-101 is designed to inhibit the stimulatory effect of CD28 activation, while preserving the immunoregulatory function of CTLA-4.5 Veloxis is developing VEL-101 as an immunosuppressant medication in its pursuit of bringing novel therapies to transplant recipients.7
Video References
- Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med. 2010;363(15):1451-1462. doi:10.1056/NEJMra0902927
- Gupta G, Womer KL. Profile of belatacept and its potential role in prevention of graft rejection following renal transplantation. Drug Des Devel Ther. 2010;4:375-382. Published 2010 Dec 1. doi:10.2147/DDDT.S10432
- Ville S, Poirier N, Blancho G, Vanhove B. Co-Stimulatory Blockade of the CD28/CD80-86/CTLA-4 Balance in Transplantation: Impact on Memory T Cells? Front Immunol. 2015;6:411. Published 2015 Aug 10. doi:10.3389/fimmu.2015.00411
- Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can't live without ... J Immunol. 2013;191(12):5785-5791. doi:10.4049/jimmunol.1390055
- Poirier N, Mary C, Dilek N, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab' antibody. Am J Transplant. 2012;12(10):2630-2640. doi:10.1111/j.1600-6143.2012.04164.x
- Kumar V, Shinagare AB, Rennke HG, et al. The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature. Oncologist. 2020;25(6):505-514. doi:10.1634/theoncologist.2019-0659
- National Library of Medicine (U.S.). (2022, May- ). A Dose Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VEL-101. Identifier NCT052384935. https://clinicaltrials.gov/ct2/show/NCT05238493
VEL-101 IS AN INVESTIGATIONAL AGENT FOR WHICH THERE IS NO MARKETING AUTHORIZATION IN THE UNITED STATES. THE SAFETY AND EFFICACY OF VEL-101 HAS NOT BEEN ESTABLISHED. PROPOSED MOA DATA ARE BASED ON IN VITRO/IN VIVO DATA.
See study publicationART-123 Clinical Program
Platinum-based chemotherapy drugs are known to cause chemotherapy-induced peripheral neuropathy (CIPN).5 Typical CIPN symptoms include pain, numbness, and/or tingling that begins in the hands and/or feet. These symptoms can impair day-to-day activities and may negatively impact cancer treatment outcomes due to reduced dosage or suspension of chemotherapy administration.
Currently, no drugs are approved in the US for CIPN prevention or treatment. To address this unmet medical need, Asahi Kasei Pharma Corp., together with Veloxis, is developing ART-123 (thrombomodulin alfa) for prevention of the sensory symptoms of CIPN in metastatic colorectal cancer patients receiving chemotherapy that includes Oxaliplatin.
References
1. Poirier N, Blancho G, Hiance M, et al. First-in-human study in healthy subjects with FR104, a pegylated monoclonal antibody fragment antagonist of CD28. J Immunol. 2016;197(12):4593-4602. 2. US National Institutes of Health, National Library of Medicine. A Dose Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of VEL-101 [NCT05238493]. https://clinicaltrials.gov/ct2/show/NCT05238493. 3. Poirier N, Mary C, Dilek N, et al. Preclinical efficacy and immunological safety of FR104, an antagonist anti-CD28 monovalent Fab' antibody. Am J Transplant. 2012;12(10):2630-2640. 4. Poirier N, Dilek N, Mary C, et al. FR104, an antagonist anti-CD28 monovalent fab' antibody, prevents alloimmunization and allows calcineurin inhibitor minimization in nonhuman primate renal allograft. Am J Transplant. 2015;15(1):88-100. 5. Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Expert Opin Drug Saf. 2004;3(6):535-546.
Let’s bring research to reality
Learn more about opportunities to partner with Veloxis.
Keep up-to-date on product pipeline and future R&D plans
Sign up for product updates and more news from Veloxis Pharmaceuticals. Your contact information will be used only for communications from Veloxis. Please review our Privacy Policy to learn more about how we use your information.